Alkanes : Study
Researchers have developed a novel method to activate alkanes using confined chiral Brønsted acids, significantly enhancing the efficiency and selectivity of chemical reactions.
- Alkanes are organic compounds that consist entirely of single-bonded carbon and hydrogen atoms and lack any other functional groups.
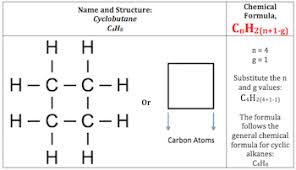
- Alkanes have the general formula CnH2n + 2 (where n is an integer).
- Alkanes can be subdivided into three groups: linear straight-chain alkanes,branched alkanes, and cycloalkanes.
- They show little chemical affinity for other substances and are chemically inert to most laboratory reagents.
- They are also relatively inert biologically and are not often involved in the chemistry of living organisms.
- Alkanes do, however, react with oxygen, halogens, and a few other substances under appropriate conditions.
- Reaction with oxygen occurs during combustion in an engine or furnace when an alkane is used as a fuel.
- Carbon dioxide and water are formed as products, and a large amount of heat is released.
- They are commercially very important, being the principal constituent of gasoline and lubricating oils and are extensively employed in organic chemistry.
- Examples of alkane include methane, ethane, propane, butane, etc.




