Mitochondrial Protein Import:
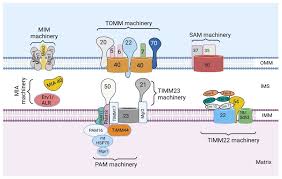
Researchers at Caltech University have uncovered new rules governing mitochondrial protein import, revising the long-standing understanding of how proteins are transported into mitochondria.
- Earlier, it was believed that mitochondrial proteins are imported only after translation is completed in the cytosol.
- Proteins were thought to fully synthesize on ribosomes before passing through mitochondrial membrane channels.
- Around 20% of mitochondrial proteins are cotranslationally imported, i.e., they are imported while still being synthesized by ribosomes.
- This mechanism mainly applies to large and structurally complex proteins that require assistance during folding.
- If these proteins fully fold in the cytosol, they risk forming irreversible structures that block import channels.
- Such proteins contain a mitochondrial targeting sequence, but this alone is insufficient for cotranslational delivery.
- A second signal is required – the first large protein domain that emerges during translation.
- This domain acts like a “code to unlock the boarding pass”, ensuring the protein is guided into mitochondria early.
- Experiments confirmed that transplanting these domains onto other proteins rerouted them for cotranslational import.
- Mitochondria are double-membraned organelles that generate ATP (adenosine triphosphate), the universal cellular energy currency.
- They originated over a billion years ago through endosymbiosis between a primitive archaeal cell and a bacterium.
- Over time, mitochondria transferred most of their genes to the host nucleus, making them dependent on the host cell for protein supply.




