Isobutanol:
The Automotive Research Association of India (ARAI) is working to explore the possibility of blending 10% isobutanol with diesel, Union Minister for Transport said recently.
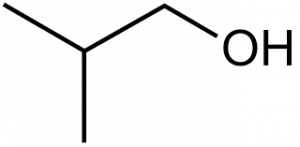
- Isobutanol , also called isobutyl alcohol, is an alcohol with the chemical formula C₄H₁₀O and one of the four isomers of butanol.
- It is a clear, colorless liquid with a characteristic odor.
- It is only moderately soluble in water.
- It is very flammable and has a flash point that is only slightly above normal room temperatures.
- Its vapors are heavier than air and can spread unnoticed along the ground.
- Skin contact, ingestion, and inhalation of isobutanol can be harmful to health.
- The compound causes skin irritation and severe eye damage, including loss of vision.
- It is used as a solvent in the flavor, fragrance, pharmaceutical, and pesticide industries and as a chemical manufacturing ingredient for products such as lacquer, paint strippers, paint primer, and craft paints.
- It is an approved food additive and is also naturally occurring in some foods and many alcoholic beverages.
- Isobutanol may also be used as a biofuel because, like ethanol, it can be manufactured from plants. It can be made from ethanol using fermentation processes.
- It possesses some favorable properties that make it an attractive fuel for internal combustion engines.
- For instance, when compared to ethanol, isobutanol features a higher heating value.
- Isobutanol is less corrosive than ethanol and is much less hygroscopic, which enables it to be transported using the existing fuel infrastructure.
- Moreover, its addition to gasoline does not distort the fuel blend’s vapor pressure to the same extent as ethanol does. All of this while having a high octane rating.




