Haber-Bosch Process:
A hundred million tonnes of nitrogen are now removed from the atmosphere and converted into fertilizer via the Haber-Bosch process, adding 165 million tonnes of reactive nitrogen to the soil.
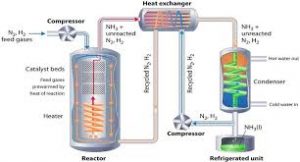
- Haber-Bosch Process is a process that fixes nitrogen with hydrogen to produce Ammonia (NH3)—a critical part of the manufacture of plant fertilizers.
- The process was developed in the early 1900s by Fritz Haber and was later modified to become an industrial process to make fertilizers by Carl Bosch.
- It is considered by many scientists and scholars as one of the most important technological advances of the 20th century.
- It is extremely important because it was the first of the processes developed that allowed people to mass-produce plant fertilizers due to the production of ammonia.
- It was the first industrial chemical process to use high pressure for a chemical reaction.
- It directly combines nitrogen from the air with hydrogen under extremely high pressures and moderately high temperatures.
- A catalyst made mostly from ironenables the reaction to be carried out at a lower temperature than would otherwise be practicable
- The removal of ammonia from the batch as soon as it is formed ensures that an equilibrium favouring product formation is maintained.
- The lower the temperature and the higher the pressure used, the greater the proportion of ammonia yielded in the mixture.




