Photoelectric Effect:
Researchers are breathing new life into the phenomenon of photoelectric effect, which is paving way for better imaging of proteins and viruses, gaining a deeper understanding of biochemical reactions and choosing new materials for next-generation electronics.
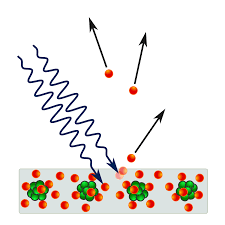
- Photoelectric Effect is a phenomenon where electrons are emitted from a material’s surface when it is exposed to light of sufficient frequency.
- When light photons hit the surface of a material, usually a metal, they transfer their energy to the electrons. If this energy is sufficient, the electrons are emitted from the material.
- The energy must be greater than the electron’s binding energy, known as the work function, for the electron to be ejected from the material’s surface.
- The excess energy from the photon, after overcoming the work function, is converted into the kinetic energy of the ejected electron.
- A material that can exhibit this phenomenon is said to be photoemissive, and the ejected electrons are called Photoelectrons.
- The effect was discovered in 1887 by the German physicistHeinrich Rudolf Hertz.
- The photoelectric effect is pivotal in understanding the quantum nature of light, as it reveals that light possesses both wave-like and particle-like properties.
- This duality is a cornerstone of quantum mechanics, illustrating how light can exhibit behaviors characteristic of both waves and discrete particles.
- The discovery and understanding of the photoelectric effect have profound implications in various scientific and technological fields, including the development of photovoltaic cells and advanced imaging technologies.




