CAR T-cell Therapy:
The clinical trial results of India’s first CAR T-cell therapy, published in The Lancet, show that it worked for nearly 73 percent of patients.
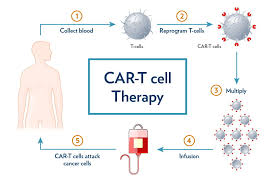
- CAR T-cell therapy, or chimeric antigen receptor T-cell therapy, trains the body’s own immune cells to identify and destroy cancer cells.
- This treatment is designed for specific types of blood cancer and is given to patients whose cancer has either relapsed or not responded to first-line treatment.
- For any CAR T-cell therapy, a patient’s immune T-cells are collected by filtering their blood.
- These cells are then engineered in a lab to add receptors that can bind with cancer cells. These cells are then multiplied and infused in the patient.
- Usually, the cancer cells are adept at evading the unmodified T cells.
- The treatment developed in India is meant for patients with two types of blood cancers that affect the B cells — acute lymphoblastic leukemia and large B cell lymphomas.
- A serious immune overreaction causing hyperinflammation and organ damage, seen in 12% of participants, resulting in at least one death.
- Low red blood cell count, reported in 61% of participants, causing fatigue and weakness.
- Thrombocytopenia: Low platelet count, increasing the risk of bleeding, reported in 65% of patients.
- Neutropenia: Low neutrophil count, seen in 96% of participants, raising the risk of infections.




