SINOVAC Covid Vaccine: China:
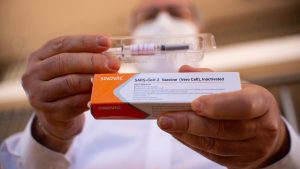
The World Health Organization approved China’s SINOVAC Covid vaccine for Emergency Use Listing, EUL. This makes SINOVAC the 8th vaccine to receive EUL from the WHO.
- WHO’s Emergency Use Listing (EUL) is a prerequisite for COVAX Facility vaccine supply and international procurement. It opens the door for the jab to be used in the COVAX program, which aims to ensure fair access to vaccines.
- The vaccine is produced by the Beijing-based pharmaceutical company Sinovac.
- The Sinovac-CoronaVac product is an inactivated vaccine (The disease-carrying virus or bacterium, or one very similar to it, is inactivated or killed using chemicals, heat, or radiation).
- Its easy storage requirements make it very manageable and particularly suitable for low-resource settings.




