Proton Exchange Membrane Fuel Cell:
The Centre for Fuel Cell Technology (CFCT) at the International Advanced Research Centre for Powder Metallurgy and New Materials showcased a mobile Proton Exchange Membrane fuel cell (PEMFC)-based backup power solution for telecom towers using a plug-and-play model.
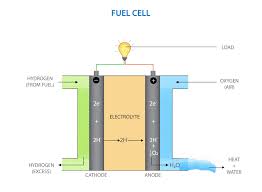
- It is an electrochemical device that converts the chemical energy of hydrogen and oxygen into electricity through a series of redox reactions.
- Unlike traditional batteries, which store chemical energy internally, PEM fuel cells require a continuous supply of hydrogen fuel and oxygen (typically from the air) to sustain the chemical reaction and generate electricity.
- The working principle involves an electrochemical reaction where hydrogen gas is fed into the anode, oxidized to release protons, which then travel through a polymer membrane to the cathode, where they react with oxygen to produce electricity and water.
- They offer an environmentally friendly solution with high power density in a compact size.
- They run on hydrogen fuel, which can be stored and transported for refuelling, and require significantly less maintenance than traditional backup power sources.




