Fischer Tropsch (FT) Process : Efficient Fuel Production
Recently discovered previously unknown self-sustained oscillations in the Fischer-Tropsch process that could someday allow for more efficient fuel production.
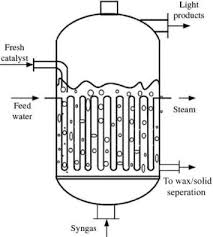
- FT process is the process where synthesis gas (H2 and CO) is converted into a mixture of hydrocarbons, oxygenates, water, and carbon dioxide.
- It was first developed in the 1920s and was named after its discoverers, Franz Fischer and Hans Tropsch.
- It involves the reaction of carbon monoxide (CO) and hydrogen (H2) gases. These gases are typically derived from various sources, including coal, natural gas, or biomass, through the process of gasification.
- Synthesis gas (syngas) is the feed material for a FT process.
- The FT reaction is usually a catalytic reaction at high temperatures and high pressure and the typical catalysts used are based on iron or cobalt.
- FT process is the catalytic polymerization and hydrogenation of CO, which produces a synthetic crude oil (syncrude).
- Syncrude is a multiphase mixture of hydrocarbons, oxygenates, and water.
- The next step is the refining of the syncrude into products that are traditionally produced from conventional crude oil, such as transportation fuels and petrochemicals.




